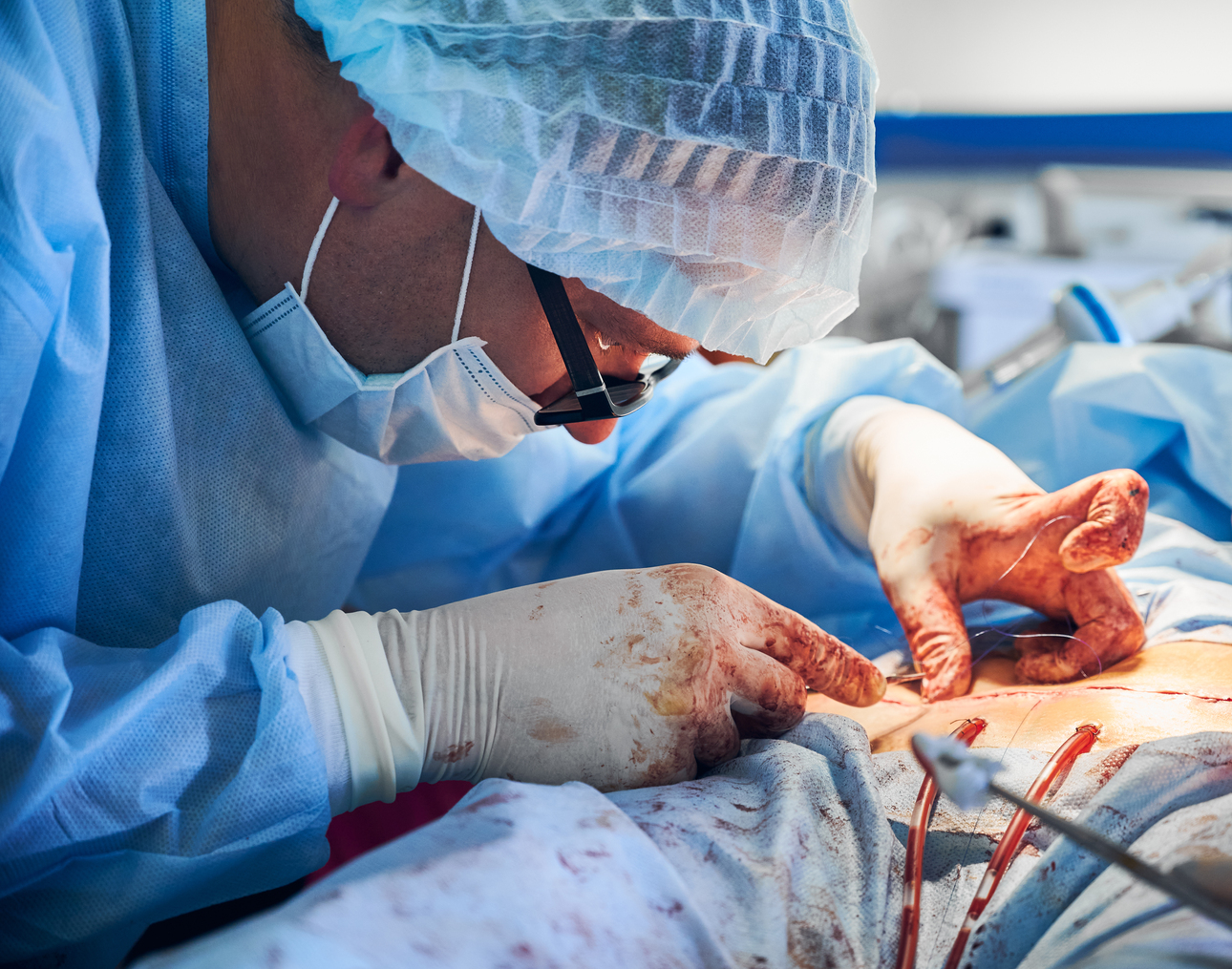
FDA: Did You Know a Pig Kidney Could Save Your Life?
The FDA has recently approved the first clinical trial to evaluate the feasibility of genetically modified pig kidney transplants.
The U.S. Food and Drug Administration (FDA) Approves First Clinical Trial for Pig Kidney Transplants in Humans
The U.S. Food and Drug Administration (FDA) has recently approved the first clinical trial to evaluate the feasibility of genetically modified pig kidney transplants in humans, known as xenotransplantation. This breakthrough offers hope to the more than 90,000 people in the United States currently waiting for a kidney transplant and could pave the way for similar initiatives globally.
The Organ Shortage Crisis
The demand for transplant organs far exceeds the availability of human donors. According to the National Kidney Foundation, more than 100,000 people in the United States are on the kidney transplant waiting list, and 17 people die every day while waiting for an organ. This disparity has driven research into xenotransplantation as a potential solution to address the organ shortage crisis.
Advancements in Xenotransplantation
Pigs are considered ideal donors for xenotransplantation due to the similarity in the size of their organs and their availability. However, one of the biggest challenges has been immediate immune rejection by the human recipient. To overcome this obstacle, scientists have used gene-editing techniques to modify pig genes, removing those that trigger adverse immune responses and adding human genes that promote organ acceptance. These advances have allowed pig kidneys transplanted into humans to maintain basic functions such as waste filtration and urine production.
Recent Cases and Results
Before the FDA’s approval of the clinical trial, experimental procedures were conducted on brain-dead patients. In one such case, a genetically modified pig kidney was transplanted into a man, and the organ functioned properly for 74 hours without signs of rejection. These preliminary results were encouraging and laid the foundation for clinical trials in living patients.
In March 2024, Richard Slayman, 62 years old, became the first living recipient of a genetically modified pig kidney at Massachusetts General Hospital. Although he was discharged without signs of rejection, he passed away nearly two months later due to an unrelated cardiac event.
More recently, in November 2024, Towana Looney, 53 years old, underwent a genetically modified pig kidney transplant at NYU Langone Health. After spending eight years on dialysis and having no human donor options due to high antibody levels, the transplant gave her a new chance at life. She was discharged 11 days after surgery and has reported a significant improvement in her quality of life.
Challenges and Ethical Considerations
Despite advancements, xenotransplantation raises safety concerns, such as the risk of zoonotic infections (diseases transmitted from animals to humans) and long-term organ rejection. Additionally, there are ethical debates surrounding animal welfare and the implications of genetically modifying pigs for human benefit. It is essential to address these concerns through rigorous research and ethical discussions to ensure the safety and acceptance of this practice.
Future Outlook
The FDA’s approval of human clinical trials marks a significant milestone in xenotransplantation research. If these trials prove to be safe and effective, genetically modified pig organ transplants could become a viable solution to the organ shortage crisis, offering hope to thousands of patients waiting for transplants. However, continued comprehensive research and addressing ethical and safety concerns are crucial before this practice is widely adopted.









LEAVE A COMMENT: